DePuy Synthes 4.5mm VA-LCP Curved Condylar Plate
In the ever-progressing landscape of orthopedic medicine, the DePuy Synthes 4.5mm VA-LCP Curved Condylar Plate stands as a significant advancement. This article provides a comprehensive examination of this medical device, its design, utilization, and potential complications. We'll consider its role in treating thighbone fractures, explore the implications of its design flaws, and discuss the responses from the medical community and regulatory bodies. This offers a valuable resource in understanding this notable medical tool.
Key Takeaways
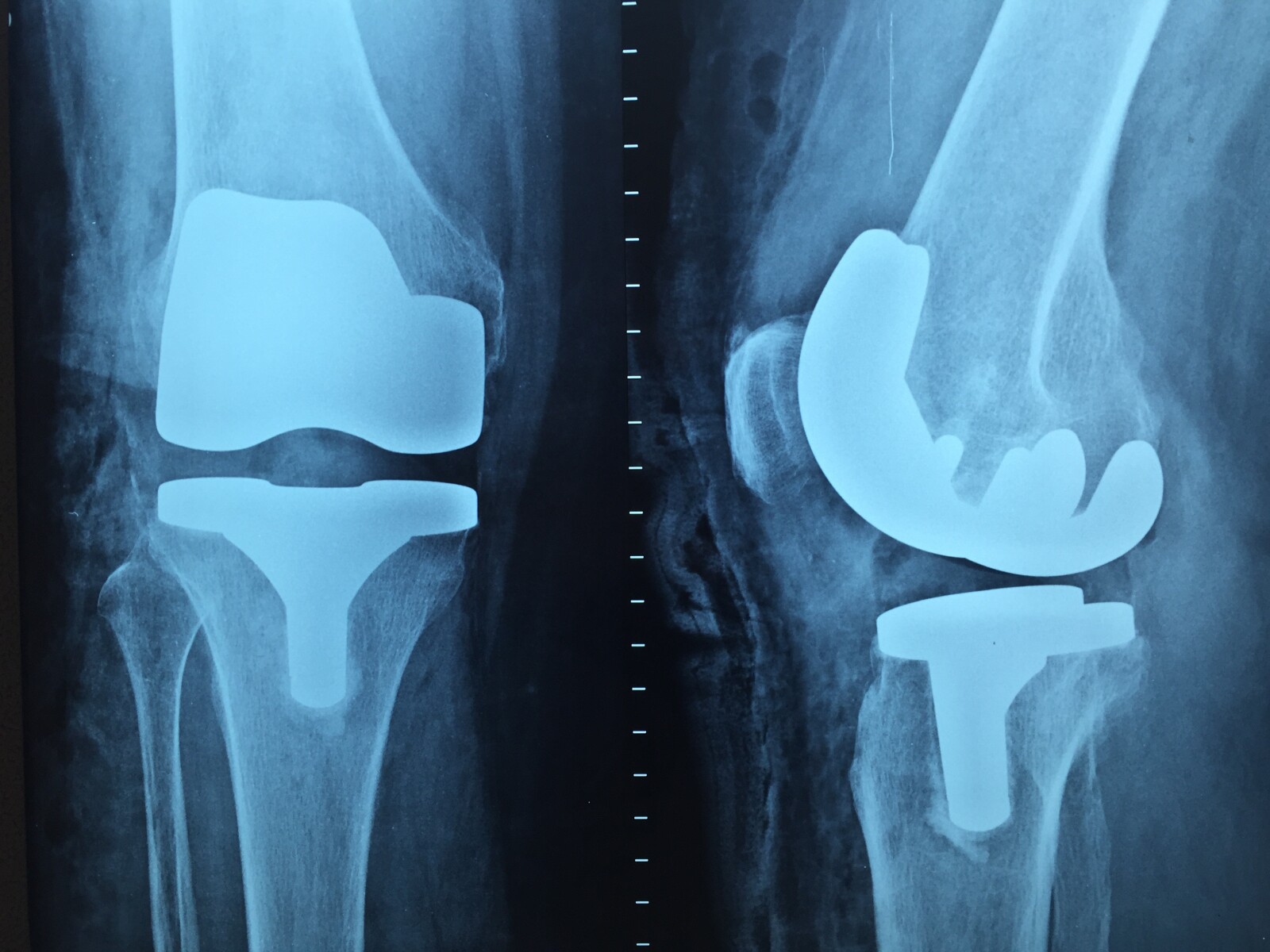
- The DePuy Synthes 4.5mm VA-LCP Curved Condylar Plate is a locking compression plate used to repair thighbone fractures above the knee.
- The most serious complication of the DePuy Synthes 4.5mm VA-LCP Curved Condylar Plate is breakage, which may require revision surgery.
- Design flaws, such as the honeycomb pattern of holes for locking screws and a weak spot in the plate's design, may contribute to plate failures.
- Studies have shown higher rates of mechanical failure for curved condylar plates compared to traditional locking plates.
Understanding the DePuy Synthes 4.5mm VA-LCP Curved Condylar Plate
Drawing from extensive research and studies, understanding the DePuy Synthes 4.5mm VA-LCP Curved Condylar Plate involves comprehending its unique design, application in treating thighbone fractures above the knee, and potential complications associated with its usage. The device, designed for orthopedic use, has revolutionized the treatment of thighbone fractures. However, it's not without risks, such as the potential for misdiagnosis or device failure necessitating revision surgery. Some studies have indicated that the specific design may contribute to these issues. This has led to a consideration of alternative treatment options, which might offer lower risk or more efficient healing. Despite these concerns, the Condylar Plate remains a widely used tool in orthopedic surgery, showcasing the balance between innovation and caution in medical treatments.
The Purpose and Design of the Condylar Plate
The DePuy Synthes 4.5mm VA-LCP Curved Condylar Plate, a medical device used in orthopedic surgery, serves two primary purposes: to stabilize and facilitate the healing of thighbone fractures above the knee. As a part of the biomechanics of the condylar plate, the design incorporates a curved shape to closely mimic the natural anatomy of the thighbone, while the locking compression technology ensures stable fixation. This engineered design is a part of the evolution of plate designs in fracture treatments, offering improved outcomes for patients. Its strategic screw placement opportunities allow for versatile fixation options. However, careful surgical planning and precise technique are essential to maximize the potential benefits of this advanced plate design.
The Role of the Plate in Thighbone Fracture Treatments
While the DePuy Synthes 4.5mm VA-LCP Curved Condylar Plate is primarily employed to stabilize and expedite the healing process of thighbone fractures, its role also extends to enhancing patient mobility and reducing the risk of future fractures. It's critical to note that this device complements advances in surgical techniques for thighbone fracture repair. Surgeons now have more precision and control during procedures, ensuring the right alignment and fixation of the fracture. Post-surgery, the plate aids in the role of physical therapy in thighbone fracture recovery. The stability provided by the plate allows patients to begin weight-bearing exercises sooner, leading to improved mobility, muscle strength, and overall recovery. Thus, the plate plays an integral part in modern thighbone fracture treatments.
The Emergence of Locking Plate Technology
Both a significant advancement and a game-changer in orthopedic surgery, locking plate technology emerged and transformed the landscape of fracture management. This innovation marked one of the key advancements in fracture treatment, offering the potential for improved patient outcomes and more streamlined surgical procedures. The benefits of locking plate technology are multifaceted. It provides increased stability, allowing for more precise alignment and positioning, thus facilitating optimal bone healing. Furthermore, it reduces the risk of hardware failure and subsequent complications. The technology's adaptability to various anatomical structures significantly broadened its application spectrum. Thus, the introduction of this technology not only revolutionized fracture treatment practices but also paved the way for future innovations in the field of orthopedic surgery.
Misuse of Locking Plates in Medical Procedures
Despite their significant benefits, misuse of locking plates in medical procedures can lead to severe complications, including plate breakage and surgical revision. The overuse of locking plates is a growing concern in modern orthopedics. It is crucial to remember that these devices are not a universal solution and their indiscriminate use can lead to unnecessary complications. Physicians need to consider patient-specific factors and explore alternatives to locking plate technology when planning their surgical approach. Novel techniques and materials are being developed to address the specific limitations of locking plates. Furthermore, training and awareness regarding the proper use and potential drawbacks of these devices are essential to prevent misuse and optimize patient outcomes.
Complications Associated With the Condylar Plate
The DePuy Synthes 4.5mm VA-LCP Curved Condylar Plate, although designed for treating thighbone fractures, can lead to numerous complications such as plate breakage and infection. Breakage of the plate often necessitates a revision surgery to remove and replace the device, adding to the patient's discomfort and recovery time. Infections, while a risk with any surgical procedure, can lead to severe health consequences if not promptly identified and treated. Other complications may include pain, bone fracture, failure of bone healing, and inability to walk or move the leg. Understanding these potential complications is crucial for healthcare providers to devise suitable treatment options, and for patients to make informed decisions about their health care.
The Necessity of Revision Surgery in Case of Plate Breakage
--v 5.2 --ar 16:9
In our ongoing discussion, we must now consider the necessity of revision surgery when plate breakage occurs, a burdensome but often unavoidable consequence. The impact of plate breakage on patient outcomes can be substantial, leading to increased pain, impaired mobility, and a prolonged recovery period. Furthermore, the breakage of the DePuy Synthes 4.5mm VA-LCP Curved Condylar Plate may necessitate a secondary surgery to remove and replace the device, adding to the physical and emotional toll on the patient. This also compounds the cost implications of revision surgery, as it includes not only the surgical procedure itself but also subsequent physical therapy and potential loss of income. Thus, the breakage of this device presents a significant issue in orthopedic medicine.
Risks of Infection in Plate Implantation Surgeries
Surgical procedures, such as plate implantation, carry inherent risks, and one of the most concerning is the potential for postoperative infection. These risks of plate infection can be due to several factors including the patient's overall health, the sterility of the surgical environment, and the postoperative care regimen. Infections can lead to serious complications, such as prolonged recovery time, additional surgeries, and in severe cases, septicemia. Hence, prevention strategies for plate infections are crucial. These may include the use of prophylactic antibiotics, stringent surgical sterility protocols, and patient education on postoperative wound care. Despite these measures, the risk cannot be completely eliminated, underlining the importance of early detection and prompt intervention in case of infection.
Common Symptoms of Plate Failures
Patients experiencing plate failure often report symptoms such as persistent pain, inability to move the affected limb, and failure of bone healing. These symptoms signal that the DePuy Synthes 4.5mm VA-LCP Curved Condylar Plate may not be functioning as intended, necessitating immediate medical attention. Factors affecting plate failures could range from surgical errors, patient characteristics, or issues with the plate itself such as design flaws. Prevention measures for plate failures involve careful patient selection, meticulous surgical technique, and appropriate postoperative care. Regular follow-ups are crucial to detect plate failures early. Additionally, advancements in plate design and material are also essential for reducing the chances of failure, thus alleviating the associated symptoms.
Investigation of FDA Reports on Plate Failures
Several reports submitted to the FDA indicate potential device failures with the DePuy Synthes 4.5mm VA-LCP Curved Condylar Plate, and these instances necessitate a comprehensive investigation to ensure patient safety. The FDA investigation aims to determine the specifics of plate failure causes, with an emphasis on incidents involving the DePuy Synthes plate. Reported failures have ranged from premature breakage to inadequate bone healing, prompting concerns about the device's design and application. A significant part of this investigation involves analyzing the situational factors leading to these failures. It is vital that such issues are thoroughly examined and addressed promptly to minimize risks to patients. The results of this investigation will shape future practices, potentially leading to design modifications or changes in surgical techniques.
Analyzing Design Flaws in the Condylar Plate
The analysis of potential design flaws in the DePuy Synthes 4.5mm VA-LCP Curved Condylar Plate is a crucial consideration, particularly given the significant number of reported device failures. One of the primary causes of plate breakage is the honeycomb design of screw holes which may weaken the overall structure. Additionally, the thin section near the plate's outer edge has been identified as a potential weak spot. These design flaws can lead to mechanical failure, requiring revision surgery and causing patient distress. Prevention strategies for plate failures include redesigning these weak points and rigorous pre-implementation testing. Regular monitoring and swift response to adverse patient outcomes are also crucial in mitigating the risks associated with such design flaws.
Research Findings on Plate Breakage Patterns
While investigating the prevalent issues related to plate breakage of the DePuy Synthes 4.5mm VA-LCP Curved Condylar Plate, researchers have uncovered specific patterns that may contribute to this significant complication. The breakage primarily occurs due to a combination of design flaws and excessive stress on the plate. The honeycomb pattern of holes for locking screws and the thin section near the outer edge have been identified as weak spots. Studies suggest that these weaknesses, coupled with the mechanical stress of bearing weight, lead to plate breakage. Prevention strategies revolve around design improvements, while treatment involves revision surgery to replace the broken plate. Understanding these plate breakage causes is crucial for enhancing patient safety and surgical outcomes.
Comparative Study of Traditional Locking Plates and Condylar Plates
A substantial body of research has been dedicated to comparing the efficacy and complications of traditional locking plates vis-à-vis condylar plates in treating thighbone fractures. In a comprehensive comparative analysis, traditional locking plates have demonstrated fewer complications and more durability in some studies. However, the DePuy Synthes 4.5mm VA-LCP Curved Condylar Plate has shown potential for improved anatomical fit and stability due to its curved design. As for long-term outcomes, both types of plates have shown varied success rates in terms of fracture healing and patient mobility. The selection between the two often depends on factors such as fracture type, patient age, and the surgeon's familiarity with the device. Ongoing research continues to evaluate the optimal application for each plate type.
The Importance of User Feedback for Drugwatch.com
Given that user feedback plays a critical role in shaping the content and services provided by Drugwatch.com, it is indispensable in ensuring the site's continued relevance and efficacy in delivering up-to-date and reliable medical information. The importance of user feedback cannot be overstated as it allows for improvements, corrections, and updates to be made based on real-world experiences and insights. The impact of user feedback thus extends beyond mere critique; it provides a crucial link between the site's offerings and its users' needs and expectations. It fosters a dynamic, reciprocal relationship that ultimately enhances the quality, accuracy, and practicality of the information provided, reinforcing Drugwatch.com's commitment to its users and their health and wellbeing.
Providing Contact Information and Seeking Assistance From Drugwatch.Com
In order to receive personalized assistance or to provide feedback about the DePuy Synthes 4.5mm VA-LCP Curved Condylar Plate, you can contact Drugwatch.com via their official website. The site offers a contact form where you can provide your full name, email, and message for feedback. This contact information is essential in seeking assistance, addressing queries, or sharing your experiences. The Drugwatch team is responsive and will get in touch with you promptly. They are committed to providing high-quality, reliable information about medical devices and health matters. By reaching out, you can gain invaluable insights, contribute to their repository of knowledge, or find legal help if you've been adversely affected by the DePuy Synthes device.
Frequently Asked Questions
What Is the Cost of the Depuy Synthes 4.5mm VA-LCP Curved Condylar Plate and Is It Covered by Insurance?
The cost of orthopedic devices, like condylar plates, may vary depending upon the provider and the specific nature of the surgical procedure. It's important to consider not only the initial cost but also the durability of the plate. Insurance coverage for such treatments is typically determined on a case-by-case basis, considering factors such as medical necessity and the specifics of an individual's insurance plan. Always consult with your healthcare provider and insurance company for accurate cost and coverage information.
Are There Alternative Treatments Available for Thighbone Fractures Above the Knee Other Than the Depuy Synthes 4.5mm VA-LCP Curved Condylar Plate?
Yes, there are alternative treatments for thighbone fractures above the knee. Plate alternatives include traditional locking plates, intramedullary nails, and external fixators. Non-surgical options, although limited, may include casting or bracing for certain types of fractures. However, the choice of treatment depends on multiple factors including the type and location of the fracture, patient's overall health, and surgeon's expertise. It is important to discuss these alternatives with a healthcare provider.
How Long Is the Recovery Period After Surgery Involving the Depuy Synthes 4.5mm VA-LCP Curved Condylar Plate?
Recovery time after surgery can vary greatly, hinging on individual health, the severity of the fracture, and plate effectiveness. As the adage goes, "time heals all wounds" - this applies to post-surgery rehabilitation too. Generally, initial healing is expected in 6-12 weeks, with full recovery extending up to a year. This includes physical therapy for restoring strength and mobility. It's crucial to follow medical advice to ensure a successful recovery.
What Is the Process for Reporting a Complication or Adverse Event Related to the Depuy Synthes 4.5mm VA-LCP Curved Condylar Plate to the Fda?
The process for reporting a complication or adverse event involves submitting a detailed account to the FDA through their MedWatch Online Voluntary Reporting Form. This includes information about the patient, the device, the adverse event experienced, and the outcome. The reporter's contact information is also required, but personal patient details are kept confidential. It is crucial to report these incidents to assist the FDA in monitoring device safety and efficacy.
If a Design Flaw Is Identified in a Device Like the Depuy Synthes 4.5mm VA-LCP Curved Condylar Plate, What Steps Does the Manufacturer Take to Correct It?
When a design flaw is identified in a medical device, the manufacturer is obliged to take immediate corrective actions. This can include issuing device recalls, enhancing design, improving post-operative care instructions, and educating healthcare providers. For instance, there were over 1,400 medical device recalls in 2019, showcasing the commitment of manufacturers to patient safety. The manufacturer also collaborates with regulatory authorities like the FDA to ensure effective resolution of the identified issues.
Conclusion
The DePuy Synthes 4.5mm VA-LCP Curved Condylar Plate, despite its proven efficacy in treating thighbone fractures, is not devoid of complications. Predominantly, plate breakage and design flaws necessitate critical examination. Research findings, comparative studies, and user feedback form integral components of understanding this device's merits and drawbacks. Hence, in-depth exploration and comprehensive understanding of this device are paramount to ensure optimal patient outcomes in orthopedic medicine.

This post has been generated by AI and was not reviewed by editors. This is Not legal advice. Please consult with an attorney.




